The current acetabular implant is a cementless 2-component cup. A metal shell for bony ingrowth + a liner to articulate with femoral head. We discuss the LINER.
Polyethylene liner (ie "poly") is the most common material type for the acetabular liner, however, ceramic or metal are also available. The Acetabular Shell provides the base for bony ingrowth, while the liner clips into the shell and articulates directly with the Femoral Head.
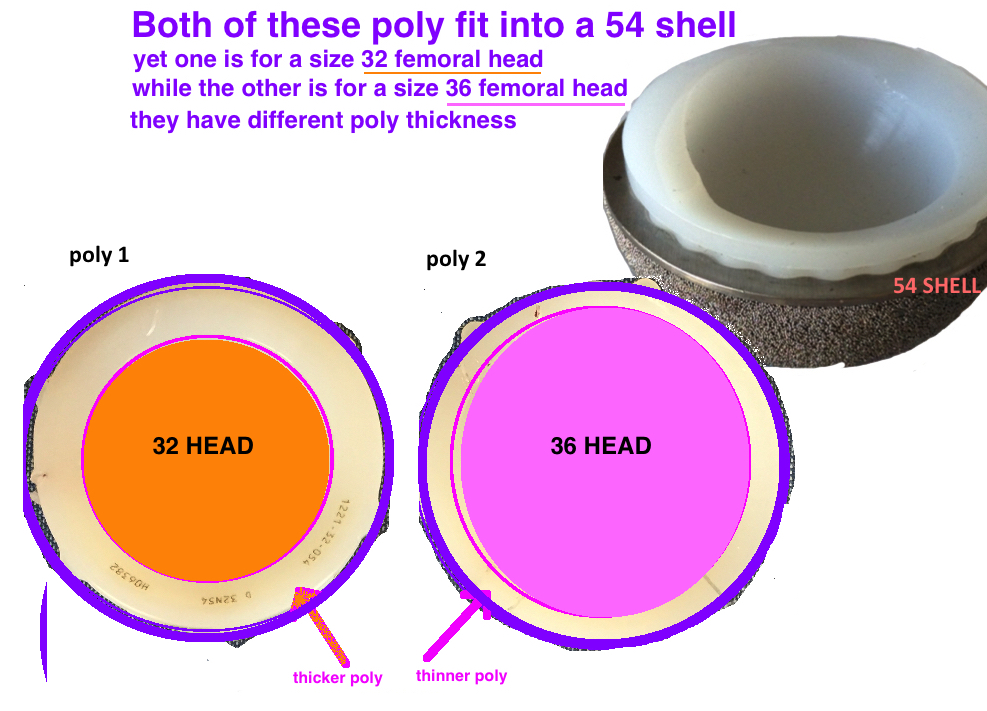
The Liner Size refers to its inner diameter = the Femoral Head Size. The poly must be at the very minimum 6 mm in thickness to prevent fracture.
The Femoral Head Size + Liner Thickness = Acetabular Shell size. Liners come in varying thickness. For example a Size 32 Femoral Head can fit into an Acetabular Cup Size 48, 50, 52 etc etc because you can get a 16 mm, 18 mm, 20 mm etc thickness poly. Importantly, there is variability in sizing between manufacturers.
Chart Showing Size of Femoral Heads that Fit Size of Acetabular Shells
Polyethylene
Poly is a long-chain polymer that’s a tough biocompatible material, and the first iteration, polytetrafluoroethylene (aka Teflon), was introduced to THA by Dr. Charnley. The original poly demonstrated high wear rates (about 0.2 mm/year) that lead to loosening from osteolysis. There was minimal poly cross-linking, and it was sterilized in air (leading to high free radical production).
Interestingly, this loosening was initially attributed to cement failure, termed “cement disease”, and this misconception drove advances in press-fit technology. Once the etiology of aseptic loosening was correctly attributed to conventional poly, poly was then seen as the leading barrier to long-term THA survival and thus multiple alternatives were developed, particularly the now notorious metal-on-metal implants.
Yet advances in poly have continued over the decades. The poly material improved to high-density polyethylene (HDPE) in the 1970s (wear 0.10 mm/year), and then to ultrahigh molecular weight polyethylene (UHMWPE) in the early 2000s, which has progressively demonstrated significantly less wear (<0.02 mm/year).
UHMWPE. There is a process to manufacturing UHMWPE. The poly is subjected to higher radiation (5-10 Mrads) that breaks poly bonds and creates free radicals that bond with other free radicals on neighboring chains to form cross-links. This is how cross-linking occurs.
The process initially creates instability, which can be problematic if the environment isn't closely regulated, for example, if there is oxygen lying around, then free radicals don't bond with each other to form cross-links but rather combine with oxygen, which propagates further free radical formation, and ultimately breaks down the poly to create "Oxidized PE". Therefore, radiation is performed in the setting of inert gas to prevent oxidation of the poly. Irradiating the PE in inert gas accomplishes two things: 1) it reduces wear by forming cross-links; and 2) it sterilizes the PE. There are techniques to sterilize the PE without cross-linking using ethylene oxide gas or gas plasma spray.
After the poly undergoes radiation in an inert gas, it is heated (via remelting, or annealing) to quench the remaining free radials (the heating allows free radicals form stable carbon-carbon covalent bonds). This process has the side effect of decreasing the crystallinity, thus decreasing toughness and tensile strength of the poly.
Some second generation UHMWPE liners are impregnated with antioxidants (such as Vit. E) to further decrease free radical breakdown after implantation.
Up to this point the poly is prepared as a solid tube of plastic. It then needs to be shaped into the poly insert that’s implanted during surgery. There are a few techniques, although direct compression molding (implant made from a mold, no machining involved) creates the lowest wear.
In summary, its believed that the summation of advancements in poly manufacturing have decreased wear by 95% compared to conventional PE.
Liner Design Types.
The standard poly liner is a neutral face hemispherical design to allow maximal range of motion (the goal is to provide a large implant ROM so that the THA falls within the motion circle allowed by native hip anatomy.
The poly can have a 10, 15, or 20° lip liner depending on the manufacturer. The “lip” is placed in the region with the greatest risk for dislocation, to provide an additional few millimeters of clearance needed for the jump distance. The poly can also be lateralized by 4 mm. In this scenario, the liner has more material on the medial side, as opposed to the apex, thereby “lateralizing” the center of rotation of the hip. This is the same as increasing offset, only it occurs on the acetabular side as opposed to the "high offset" stem. This usually also results in adding length but it is negligible. This liner can be used in cases of protrusio or revision cases when the goals are to increase stability by restoring tension on the soft tissues (particularly the abductor complex).
There are also liners used in revision cases when there is a higher risk of dislocation. This includes a dual-mobility and a constrained liner. Dual-mobility is essentially a bipolar head (as used in some hemi cases) within a cup. A constrained liner occurs when the femoral head gets locked into the poly.