Bone loss is a major challenge with revision of the femoral implant. In the face of significant bone loss, achieving fixation via press fit technique requires different implants, depending on bone loss severity.
Geometry of Revision Stems. Revision stems are extensively coated (entire stem allows for ingrowth) because bone loss primary occurs in the metaphyseal region after explanting a prior stem. Many of these stems are modular to allow the surgeon to adjust length, offset and version. To prepare for implantation, these implants require proximal broaching and distal reaming.
Extensive-Coated Stem: Biologic fixation occurs in the metaphysis and diaphysis. A well-fixed stem will have a "spot weld" at the tip of the stem. The benefit of extensive coating is that metaphyseal bone loss is common in revision cases, and therefore is not reliable site for biologic fixation. Diaphyseal fixation avoids this problem, however, because all of the force is transmitted to the area of fixation, the bone proximal to the fixation (ie the metaphyseal bone) will undergo stress shielding (it does not experience stress and therefore the bone will be resorbed, "use it or lose it"). The stem geometry also contributes to the degree of stress shielding. Because a stiffer material will bear more stress, the stiffer stem will create more stress shielding. The stiffest type of extensive-porous stem is Cobalt-Chrome (not titanium), round, solid (no flutes, no slots, no splines), and a large diameter (> 16 mm).
Wagner Design: achieve more of a metaphyseal-diaphyseal junction fixation, tapered with a round conical geometry (some have cutting flutes for rotational stability). The more distal fixation allows for use in revision cases.
Trabecular Metal is a highly porous metal which increases friction against cancellous bone to improves initial stability and encourages rapid and extensive bone ingrowth.
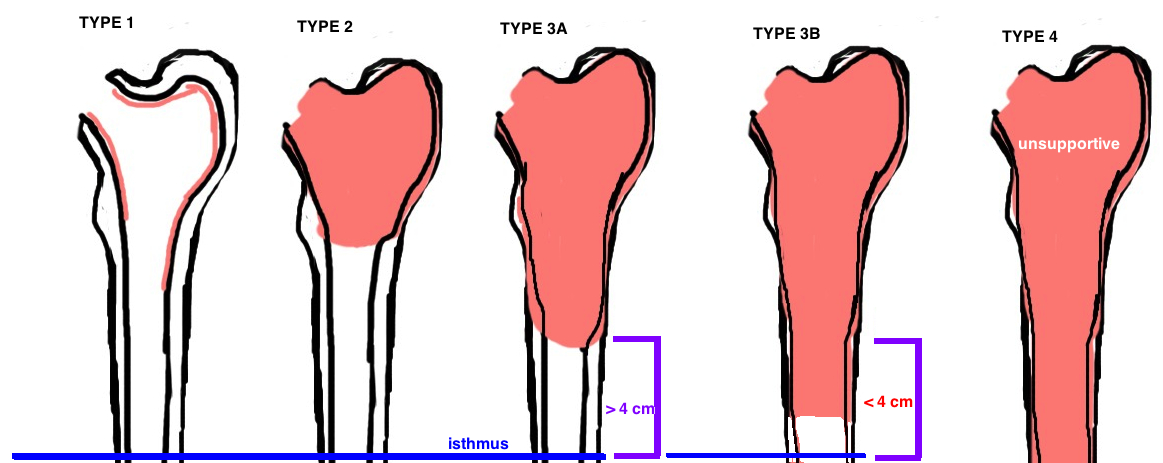
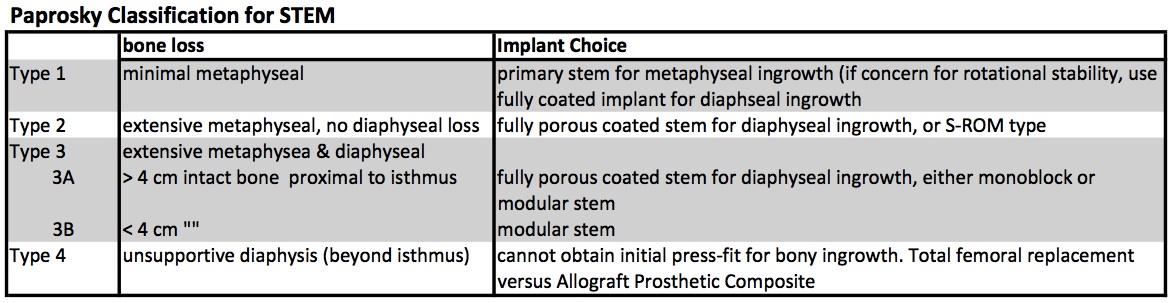
The Paprosky Classification was established to grade the severity of bone loss, which in turn guides treatment (different grades of bone loss require different implants to achieve stability). [1, 2].
Revision based on Paprosky Classification
Type 1: There is sufficient proximal bone to support any implant and therefore a primary stem (metaphyseal ingrowth) is sufficient (using a double wedge taper design which provides the best proximal fill), altought there may still be compromised rotational stability and thus many surgeons elected for a fully porous stem (diaphyseal ingrowth).
Type 2: there is insufficient metaphyseal bone stock to support the stem, thus the implant must have some diaphyseal porous coating for ingrowth.
▪ Extensively Porous-coated Cylindrical stem (also referred to as the “monobloc” or diaphyseal porous-coated). Proximal fixation isn’t possible, yet there is good diaphyseal bone for fixation, by extending the porous coating distally, ingrowth will occur in the diaphysis where there is bone. This design is successful (3-5% revision rate at 15 years). [3] [4]
▪ Porous-coated proximal sleeve that is coupled with a smooth stem containing splines for rotational stability (the S-ROM type by DePuy or the Emperion by S&N). This design requires enough metaphyseal bone stock for ingrowth, however, the modularity between proximal and distal components allows for management of significant mismatch in bone stock between the metaphysis and diaphysis [5]. The proximal “sleeve” contains a standard or a calcar-replacing option. The sleeve has “step cuts” to convert shear force into compressive force. This design promotes proximal ingrowth, it reduces the risk of stress shielding as seen in fully-porous coated stems. This design is best for revising undersized implants or early loosening. The distal stem component is variable in length and can be curved to follow the natural femoral bow. It contains splines for rotational control and a coronal split (“slot”) to reduce stiffness (and thus reduce thigh pain). The Emperion by S&N has a “bullet tip” to further decrease pressure on the cortex and reduce thigh pain (it contains porous-coat plus Hydroxyapatite coating). The biggest concern with the S-ROM type device is the extra modularity which enables micromotion at this additional joint (stem-sleeve) allowing for metal debris. This micromotion can be mitigated in part by increasing the stem diameter to fully engaging the distal femur.
Type 3A: no metaphyseal bone stock, some deficient proximal diaphyseal bone stock. Requires diaphyseal fixation. While standard fully coated stem is an option, good results have also been shown with the Modular stem designs.
▪ Extensively Porous-coated Cylindrical stem (also referred to as the “monobloc” or cylindrical diaphyseal porous-coated cobalt-chromic stem).
▪ Porous-coated proximal sleeve that is coupled with a smooth stem containing splines for rotational stability (the S-ROM type by DePuy or the Emperion by S&N). This cannot however be used for Type 3B [6].
▪ Wagner Modular Stem. This modularity separates the two goals of surgery, and allows the surgeon to work on them separately. All fixation is achieved distally (in contrast to S-ROM), with the goal of bypassing the proximal deficient bone. Then the proper length, offset, and version is achieved proximally. Then the two are connected. Basic design is Tapered, Fluted Cylindrical Stem. Axial stability is achieved by wedging the taper. Rotation stability is achieved with the flutes. Its made of titanium to reduce stress shielding. This design is the work-horse of revision THA in Europe for the past two decades before making it to the US [7]. Good clinical survivorship [8] [9].
Type 3B: even less diaphyseal bone stock (< 4 cm intact diaphysis) yet the femoral isthmus remains supportive. The Modular stem designs are the best option.
▪ Wagner Modular Stem [10] is cylindrical and tapered.
Type 4: without any supportive diaphysis, there is no way to obtain initial press-fit to allow for bony ingrowth. Therefore these cases are incredibly challenging and typically require a total femoral replacement or APC (allograft-prosthetic composite).
References
1. Engh, C.A., P. Massin, and K.E. Suthers, Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res, 1990(257): p. 107-28.
2. Valle, C.J. and W.G. Paprosky, Classification and an algorithmic approach to the reconstruction of femoral deficiency in revision total hip arthroplasty. J Bone Joint Surg Am, 2003. 85-A Suppl 4: p. 1-6.
3. Engh, C.A., Jr., et al., Extensively porous-coated femoral revision for severe femoral bone loss: minimum 10-year follow-up. J Arthroplasty, 2002. 17(8): p. 955-60.
4. Weeden, S.H. and W.G. Paprosky, Minimal 11-year follow-up of extensively porous-coated stems in femoral revision total hip arthroplasty. J Arthroplasty, 2002. 17(4 Suppl 1): p. 134-7.
5. Christie, M.J., et al., Clinical experience with a modular noncemented femoral component in revision total hip arthroplasty: 4- to 7-year results. J Arthroplasty, 2000. 15(7): p. 840-8.
6. Bolognesi, M.P., et al., Comparison of a hydroxyapatite-coated sleeve and a porous-coated sleeve with a modular revision hip stem. A prospective, randomized study. J Bone Joint Surg Am, 2004. 86-A(12): p. 2720-5.
7. Wagner, H., [Revision prosthesis for the hip joint in severe bone loss]. Orthopade, 1987. 16(4): p. 295-300.
8. Bohm, P. and O. Bischel, The use of tapered stems for femoral revision surgery. Clin Orthop Relat Res, 2004(420): p. 148-59.
9. Richards, C.J., et al., Femoral revision hip arthroplasty: a comparison of two stem designs. Clin Orthop Relat Res, 2010. 468(2): p. 491-6.
10. Paprosky, W.G., N.V. Greidanus, and J. Antoniou, Minimum 10-year-results of extensively porous-coated stems in revision hip arthroplasty. Clin Orthop Relat Res, 1999(369): p. 230-42.