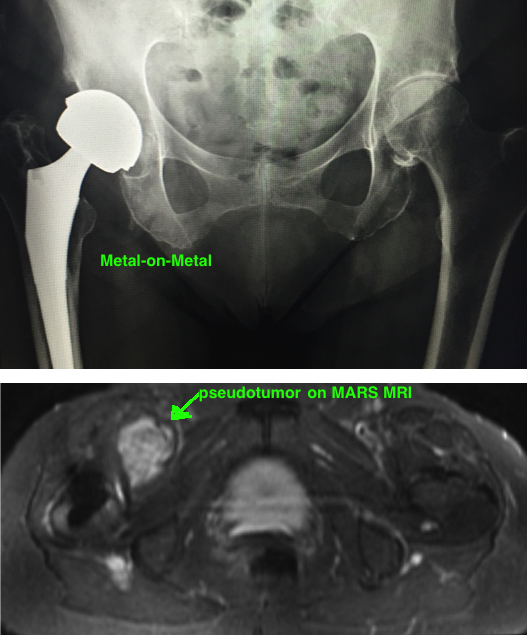
The history of Metal-on-Metal (MoM) implants dates pre-Charnley, with the McKee-Farrar implants, which fell out of favor due to early loosening and subsequently paved the road for Charnley’s metal-poly design. Yet these early MoM implants showed binary results: either failure due to early loosening or excellent long-term survivorship > 20 years. In fact, 110 of these hips were reexamined in 1990s and showed no signs of loosening [1]. The promise of these findings (the potential for superior longevity with MoM) at a time when poly implants were failing because osteolysis (a result of poor quality PE) ushered in a new era of MoM. Improved technology in biologic fixation and implant manufacturing suggested a promising low-wear alternative to metal-on-poly implants, with the hope of improving wear rates and overcoming the barrier to long-term survivorship. MoM quickly became incredibly popular in the USA, with over 50% of THA using this combination as recently as 2006. As we know, the story doesn’t end well.
Registry data (out of Europe) started showing a 2-3x higher failure rates compared to metal-on-poly. These failures were correlated with significant adverse tissue reaction from the metal particles generated by wear [2] [3]. While the overall wear rates are lower than metal-on-poly, the metal debris is considerably more reactive, and can lead to macroscopic muscle necrosis (abductor injury is particularly concerning), significant osteolysis, and large sterile cystic masses (called “pseudotumors”). Metallosis is defined as macroscopic staining, necrosis/fibrosis of periprosthetic tissue, and is associated with solid soft tissue masses, aseptic cysts, and significant soft tissue necrosis. The terms ARMD (Adverse Reaction to Metal Debris) or ALTR (adverse local tissue reaction) are similar broad terms to encapsulate these soft tissue lesions [4]. The end result is that many designs were recalled and MoM has fallen out of favor.
Mechanics of Metal Wear.
The success of metal-on-metal devices depends on a fluid-film layer that develops between components when the space is between 100-200 μm. This film provides continuous lubrication and dramatically reduces wear (hip simulators showed encouraging wear rates < 10 μm year). Yet, outside the lab, real issues like improper sizing or cup malpositioning leads to intermittent disruption of this film layer causing spikes in friction causing spikes in torque forces causing motion at the head-neck junction causing wear called “trunionosis”. This wear occurs through fretting and crevice corrosion (not abrasion).
Fretting: this is a mechanical wear from repetitive surface motion. It occurs at any junction between two contacting surfaces.
Pitting corrosion: Stainless steel and Titanium implants withstand corrosive wear by creating a “passive layer” which a non-reactive film of chromium oxide that forms when a metal is exposed to oxygen. Yet when this surface layer is depleted by Fretting, the metal is exposed directly to the free radicals within the aqueous environment causing a chemical reaction that leads to breakdown of the metal.
Interestingly the majority of wear in MoM is believed to occur at the head-neck junction (trunion), not at the femoral-acetabular articulation where other forms of wear originate. This theory is supported by comparing the metal resurfacing hips (which lack a head-neck junction) with THA, and which showed low ion levels in resurfacing hips despite large size femoral heads [5] [6] [7]. Of note, metal debris is still generated in resurfacing hips which indicates that wear occurs at multiple sites in a THA.
The wear generates significantly more particles (13-500x more) and smaller in size (0.1-0.5 μm), and these particles attract lymphocytic reaction (unlike the macrophages in poly wear).
CAUSE OF TISSUE DAMAGE.
There are two causes of this significant tissue reaction.
1) Cytotoxic local tissue effect of metal (accounts for vast majority)
2) Hypersensitivity reaction (especially seen in patients that experience groin pain without elevated metal ions) [8]. Diagnosis is the major problem because is no good test for hypersensitivity, (skin patch testing or lymphocyte transformation testing are questionable). There is no direct evidence to link patients with metal allergy to higher failure or revision of TJA, and therefore the association between symptoms and allergy is only theoretical, and therefore the diagnosis of metal hypersensitivity in the face of persistent pain and synovitis is a diagnosis of exclusion. There is a much higher chance of indolent infection and therefore full infectous work up (esr, crp, joint aspiration, possibly repeat aspiration or alpha-defensin), no need to obtain metal ion levels because they are typically elevated in patients with normal functioning implants. Patch testing and in vitro lymphocyte testing can be ordered to look for hypersensitivity, however, they are not proven to be reliable tests. [9, 10].
The key to determining the severity of an ALVAL (aseptic lymphocyte-dominated vasculitis associated lesion, which appears as a type IV hypersensitivity reaction in 0.3% of patients, more in females) lesion is multifold, different from an ALTR (adverse local tissue reaction). Obtain cobalt and chromium levels. If elevated, then repeat the test. If remains elevated then get soft tissue imaging. Currently get annual follow up for any symptomatic patient. Also annual follow up for any asymptomatic patient with a recalled hip, or a THA with a head >36 mm. Cobalt is certainly the more toxic of the two metals. Not all patients respond to the same levels of metal ions, each patient has a different threshold before they begin to demonstrate symptoms of metal hypersensitivity.
Workup.
Knowing that MoM hips can potentially lead to very severe complications, how should surgeons approach these patients, and when should they intervene? Should all MoM THA be revised? Its believed that significant ARMD occurs in 1% of cases within 5 years. Therefore revision everyone subjects a lot of patients to unnecessary surgery, however, not revising problematic MoM THA in a timely manner places patients at progressively higher risk for long-term complications.
Currently, not all MoM hips are revised, and the decision to revise is based on a number of variables. Importantly, there is not a single good test to detect problematic MoM hips, there is not a great predictive model. The decision to revise is based on a number of variables (implant type, metal ion levels, symptoms, cup position), which help to risk stratify patients.
Metal Ion levels. There is no direct correlation between blood ion levels and metallosis (and this is probably the biggest issue to date with diagnosis of metallosis) [11-13]. Gross features of metallosis typically appear when blood cobalt metal ion levels >20 ppb in the blood, or even >17 ppb [14]. Yet ARMD can certainly occur in patients under these levels.
Therefore, can metal ion levels be used to make decisions about revision? In the UK, the FDA-equivalent MHRA (Medicines and Healthcare Products Regulatory Agency) recommends that doctors obtain metal ion levels every 3 months, and if the patient is both symptomatic and levels >7 ppb…then revision. To confuse the matter further ARMD can occur secondary to hypersensitivity at very low ion levels. Elevated ion levels may affect patients beyond their hip replacement, and case reports exist of neurologic and cardiac morbidities associated with high levels of metal ions [15]. To date, there are no studies correlating metal ions with carcinogenesis [16], although it remains a concern due to the effect of cobalt on DNA in laboratory studies and some argue that the over numbers are not high enough to detect cancer risk if it exists [17]…and for this reason MoM is avoided in women of childbearing age).
Symptoms. Clinical symptoms of groin pain are suggestive of loosening, and trendelenburg gait is suggestive of abductor injury, with often indicate ARMD. ARMD can also occur in symptomatic patients, despite low levels of metal ions. Therefore, even in patients with MoM and low metal ion levels, the next step is to obtain a MARS MRI (metal-artifact reducing sequence magnetic resonance imaging) to look for signs of pseudotumor (which is indicative of ARMD). It is always important to rule out infection in any case of painful THA. When obtaining an aspiration, a manual cell count must be performed because metal debris is mistaken for WBCs by the counting machine causing the number to be falsely elevated.
What about asymptomatic patients with elevated ion levels? ARMD certainly can occur in asymptomatic patients, one study found pseudotumors in 60% of asymptomatic, and 57% of symptomatic patients, indicating that symptoms alone is not reliable [18].
Other patient risk factors include obesity and female gender (possibly related to increased risk of hypersensitivity).
Implants. Certain implants have higher rates of metallosis (such as the ASR by DePuy, Durom by Zimmer, Recap by Biomet). Additionally, all implants are at increased risk for metallosis with malpositioning, specifically high inclination angle (> 50°) which causes edge loading, and increased combined anteversion (> 40°) which causes posterior impingement, both of which causes micro-separation and thus disruption of the fluid film layer. Other implant related risk factors is a large femoral head size in MoM THA (>28 mm), or a small head size in MoM hip resurfacing (< 50 mm).
Treatment.
When determining treatment, first distinguish between Asymptomatic and Symptomatic Patients, then Risk Stratify based on a) metal ion levels, b) cup positioning, c) signs of osteolysis, and d) implant track record.
Revision surgery is recommended for signs of ARMD. Studies suggest that pathology only worsens with time, and therefore revision of components should be performed in a timely manner.
REFERENCES
1. Jacobsson, S.A., K. Djerf, and O. Wahlstrom, Twenty-year results of McKee-Farrar versus Charnley prosthesis. Clin Orthop Relat Res, 1996(329 Suppl): p. S60-8.
2. Porat, M., et al., Causes of failure of ceramic-on-ceramic and metal-on-metal hip arthroplasties. Clin Orthop Relat Res, 2012. 470(2): p. 382-7.
3. Ebramzadeh, E., et al., Failure modes of 433 metal-on-metal hip implants: how, why, and wear. Orthop Clin North Am, 2011. 42(2): p. 241-50, ix.
4. Haddad, F.S., et al., Metal-on-metal bearings: the evidence so far. J Bone Joint Surg Br, 2011. 93(5): p. 572-9.
5. Steffen, R.T., et al., The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br, 2008. 90(4): p. 436-41.
6. Mabilleau, G., et al., Metal-on-metal hip resurfacing arthroplasty: a review of periprosthetic biological reactions. Acta Orthop, 2008. 79(6): p. 734-47.
7. Pandit, H., et al., Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br, 2008. 90(7): p. 847-51.
8. Campbell, P., et al., The John Charnley Award: a study of implant failure in metal-on-metal surface arthroplasties. Clin Orthop Relat Res, 2006. 453: p. 35-46.
9. Anand, A., F. McGlynn, and W. Jiranek, Metal hypersensitivity: can it mimic infection? J Arthroplasty, 2009. 24(5): p. 826 e25-8.
10. Luetzner, J., et al., Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res, 2007. 461: p. 136-42.
11. Williams, D.H., et al., Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am, 2011. 93(23): p. 2164-71.
12. Steele, G.D., et al., Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty, 2011. 26(6 Suppl): p. 14-8.
13. Kwon, Y.M., et al., "Asymptomatic" pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty, 2011. 26(4): p. 511-8.
14. De Smet, K., et al., Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am, 2008. 90 Suppl 4: p. 202-8.
15. Tower, S.S., Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am, 2010. 92(17): p. 2847-51.
16. Keegan, G.M., I.D. Learmonth, and C.P. Case, Orthopaedic metals and their potential toxicity in the arthroplasty patient: A review of current knowledge and future strategies. J Bone Joint Surg Br, 2007. 89(5): p. 567-73.
17. Visuri, T., et al., Cancer risk after metal on metal and polyethylene on metal total hip arthroplasty. Clin Orthop Relat Res, 1996(329 Suppl): p. S280-9.
18. Hart, A.J., et al., Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia. J Bone Joint Surg Br, 2009. 91(6): p. 835-42.