Aseptic Loosening of implants is caused by Osteolysis.
Osteolysis is bone resorption caused by the body’s response to particulate debris generated as the THA implant wears out.
Particulate debris arises from motion between any two components of the prosthesis, for example: a) the femoral head and the acetabuluar liner, b) the morse taper of the head-neck junction of the femoral stem, or c) the backside of liner and shell of the acetabulum.
This debris is mistaken as a foreign invader and stimulates a host response. Metal, poly, or cement particles can all trigger osteolysis, albeit different types of reaction.
Osteolysis is important because it is the bone that integrates with the implant that resorbs and leads to implant loosening and/or periprosthetic fractures.
Historical Perspective: Osteolysis was first described by Harris in 1976 and it was attributed to “cement disease” [3], because it was observed around the femoral component, and this was what started the drive for cementless implants. Yet after significant R&D, and development of cementless implants, osteolysis was still seen around the implants [4], and the histology was similar between cemented [5] and cementless implants [6]. Surgeons then looked for another cause of osteolysis and recognized that it was produced by wear particles.
Stages of Osteolysis
1) Debris production (ie poly wear) is the initial stage (we talk about metal debris in a separate section because it behaves totally differently, see section). Particulate debris in THA is produced by Abrasive and Adhesive wear (whereas TKA produces delaminating wear: small fissures form within the poly leading to larger particles that are often to big to stimulate an immune response).
▪ Adhesive wear is two surfaces bonding together causing the softer material to “peel” off as a thin film onto the harder surface during motion.
Volumetric wear is a specific type of adhesive wear, and it occurs as the femoral head articulates with the cup liner, and the amount of wear is proportional to the femoral head radius squared (therefore larger femoral head = more wear..this is why the initial Charnley implants, which used conventional poly, used a size 22 femoral head). Linear wear is caused by focused stress on a isolated part of the poly due to abnormal loading.
▪ Abrasive wear occurs when a harder surface (which is never completely smooth) cuts or ploughs through a softer surface, like a cheese grater. Both cause particle formation. Most wear occurs superiorly in the cup (or at the rim in cases of impingement).
The conventional PE wear from articulating with a Cobalt-chrome head is 0.10 mm/year.
The ultramolecular weight poly (UMWPE, also known as highly-crosslinked poly) wear is about 0.02 mm/year, 5x less. What is the difference between conventional and UMWPE? The quality of PE depends on the consolidation of PE (the shape is formed using compression molding), the preparation (irradiation causes extensive cross-linking which decreases susceptibility to oxidation and wear), and the packaging (poly is sterilized in inert gas to prevent free-radical formation (although it can oxidize over time within the body, which is the reason some poly contains Vit. E), which theoretically eats up the free radicals generated with oxidation) [7] [8].
2) Immune Reaction & Bone Loss.
The debris then travels within the joint fluid and triggers an immune response at any area of bone that is in contact with the fluid.
This is an important concept when considering whether to place acetabular screws as this hardware effectively increases the surface area of bone in contact with synovial fluid…referred to as the “effective joint space” [5]. This concept also highlights the value of circumferential porous coating in the femoral stem, because it reduces the effective joint space by causing bone ingrowth and blocking the path of joint fluid around the implant.
Poly debris activates macrophages to initiate an inflammatory response by releasing the standard cytokines (ie TNF-alpha, RANKL, IL-6, and IL-1) that stimulate osteoclast activity, inhibit osteoblast activity, and promote progressive bone loss around the prosthesis. Osteolysis is typically progressive. Its often asymptomatic until catastrophic failure, which occurs in the form of sudden implant loosening or an acute periprosthetic fracture .
Because osteolysis is a chronic issue that causes acute complications, it must be monitored with serial radiographs for progressive changes. Signs of large lytic lesions, significant progression, or loose implants are the indications for revision surgery (even in the absence of symptoms).
Clinical correlate: Plain x-rays typically underestimate the size of osteolytic lesions, with lytic lesion only visible on x-ray once 20-30% of acetabular bone loss has occurred [9].
3) Implant Loosening.
There is no universal definition of loosening. Many consider x-ray evidence (progressive radiolucency or implant migration) sufficient to diagnose loosening, while some require clinical changes to be necessary for diagnosis. Lets consider the clinical and radiographic signs.
If there is rapid osteolysis or bone disintegration then be suspicious of infection.
clinical correlate: A common symptom of aspetic loosing is “start-up” pain - pain with the first steps, which gradually resolves, suggesting that the implant is loosen and then settles into a more stable position during weight bearing.
X-ray. The femur and pelvis have been divided into zones called Gruen Zones, which help to identify areas of osteolysis.
In general serial x-rays are performed, and changes in implant positioning like stem subsidence, provides the best evidence. Radiolucent lines that progress >2 years after surgery, or new radiolucent lines over 1 mm are significant signs of loosening. Thinning is not the only sign to look for. Focal areas of high cortical density (radiopaque lines), especially around the collar or at the end of the stem, indicate non-uniform stress (ie pedestal formation), which suggest loosening [10][11]. But remember that not all cortical thinning is osteolysis (age-related thinning, or stress shielding also cause thinning). Also, in a cementless implant, a “spot weld” found at the distal end of the stem is a sign of stable fixation. In the acetabulum there are 3 zones of radiolucency, however, radiolucencies isolated to one or two zones is less specific. Leopold et al found nonprogressive lucencies in over 50% of revised components that were performing well clinically.
If there is rapid osteolysis or bone disintegration then be suspicious of infection.
Femoral component migration is best evaluated by comparing the position of the stem relative to the calcar on serial x-rays. Of note, many press-fit stems demonstrate subtle early subsidence (<5mm) from their initial position that allows for integration without problem.
Acetabular component migration (superior or medial) should similarly be examined. A loose acetabulum may also demonstrate changes in version or inclination. The acetabular component should have circumferential radiolucent line >2 mm or implant migration.
Cement Implants. In cemented implants debonding or cement fracture will precede loosening (cement implants can loosen by fatigue fracture where cracks propagate into pre-existing pores of the cement mantle). However debonding doesn’t always cause loosening. For example, a supero-lateral lucency around the stem < 2 mm does not indicate loosening. Different with cemented implants. Changes at the bone-cement interface can just be remodeling rather than loosening, however, radiolucency at the implant-cement interface is loosening.
Look for complete radiolucent line and implant migration. An incomplete radiolucent line of 50-99% implant is only possibly loose. Progressive radiolucent line around cement-bone interface is probably loose. If radiolucency is <50% is unlikely loose if nonprogressive. The loosening rates for a cemented femur are impressively low, going back to the Charnley stem, survivorship at 20 years was over 95%, at 25 years about 89% and at 30 years about 82%.
Treatment.
While osteolysis is the primary cause of loosening, infection must be part of the differential diagnosis. Inflammatory markers ordered, and an aspiration if warranted.
The decision to operate and the type of revision surgery is controversial. Does all osteolysis need surgery? A loose implant should be revised. Symptomatic osteolysis should be revised. But the need for surgical intervention for osteolysis around a stable implant in an asymptomatic patient is less clear. The decision to operate on a hip depends on a few variables: rate of progression (delta); location of osteolysis, type of implant, activity level and age of the patient.
Studies examining the timing for surgical intervention [12 - 15] offer some guidelines. Even a few small areas of integration can keep a cup stable and thus patients remain asymptomatic despite expansile lesions [12] [15]. Yet progressive osteolysis will continue to reduce the area of integration until failure inevitably occurs. Thus, in general, lesions that progress over 3-6 month period should be revised. Femoral osteolysis similarly remains asymptomatic until extensive synovitis occurs or impending fracture.
Femoral Implant Revision.
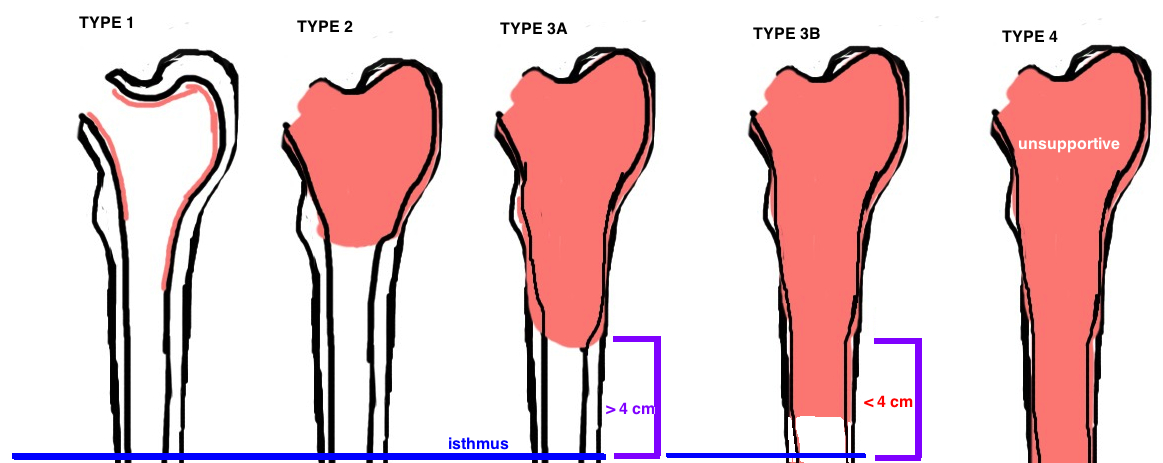
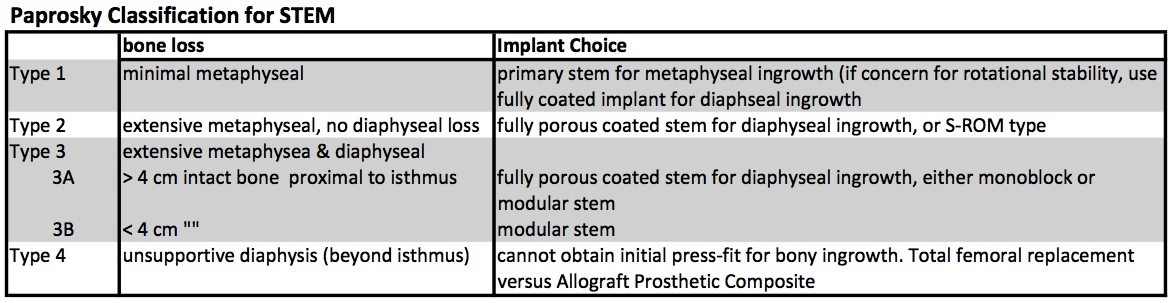
Osteolysis causes different degrees of bone loss. The Paprosky Classification was established to grade the severity of bone loss, which in turn guides treatment (different grades of bone loss require different implants to achieve stability).
Proxmially coated stems (primary implants) can be used if sufficient metaphyseal bone stock for ingrowth. Fully-coated stems (cylindrical monoblock) are used for deficient metaphyseal bone stock but decent diaphyseal bone (type 2, type 3A), however, there were high failure rates if used in cases with notable deficiency in diaphyseal bone stock. Fluted, Tapered Stems (Wagner-type) either monoblock or modular are successful for type 3B, and some type 4. Modular Wagner-type stems have grown in popularity because adjustments can be made if the final implant subsides more than the trial implant. Oncology prosthesis are often needed in type 4 implants when the bone stock is deficient past the isthmus of the femur.
Acetabular Implant Revision.
Asymptomatic acetabular osteolysis maybe be treated with a) implant revision or b) implant retention with bone grafting of the defect and head/liner exchange; or c) no surgery, close monitoring. Symptomatic acetabular osteolysis and gross loosening of the cup is treated with full component revision.
Similar to the femoral side, there is a Paprosky Classification for Acetabular Osteolysis, which grades the severity of bone loss and guides implant choices. Different grades of bone loss require different implants to achieve stability.
Head/liner exchange: considered if the components are well aligned [16]. Yet despite well aligned components there is still a 10% re-revision rate for liner exchange [17] vs. 2% re-revision rate after the cup is revised [18]. Yet other studies have found less significant differences in revision rates when the implants are well positioned [19]. In cases of malpositioned components, which may be accelerating wear, complete implant revision is probably indicated.
Cup Revision: Many surgeons will fully revise a cup with significant osteolysis, but all surgeons will revise a malpositioned cup with osteolysis and any loose cup. The cup is loose if there is >2 mm of migration observed on serial x-ray, or if there are any signs of rotational changes, any screw breakage, or a radiolucent line (>1 mm) seen in all 3 zones [20] [21].
If the rim is supportive (type 1) or partially supportive (type 2 - over 2/3 rim intact, 50% contact with bone), then a standard cup can be used (consider multi-hole or high porous metal for better scratch fit). Augments or cement + rebar can be incorporated to improve stability.
Special implants (i.e. Triflange cup, or cup-cage) are needed when the rim is unsupportive (type 3) causing the implant to rock up and roll out posteriorly (type 3A) or roll up and in causing a superior medial defect (type 3b).
It is most significant factor limiting longevity of THA. Revision for loosening is 4x higher than next leading cause (dislocation at 13.6%), and its particularly problematic in younger patients [2].
REFERNECES
1. Bozic, K.J., et al., Risk of complication and revision total hip arthroplasty among Medicare patients with different bearing surfaces. Clin Orthop Relat Res, 2010. 468(9): p. 2357-62.
2. Bozic, K.J., et al., The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am, 2009. 91(1): p. 128-33.
3. Harris, W.H., et al., Extensive localized bone resorption in the femur following total hip replacement. J Bone Joint Surg Am, 1976. 58(5): p. 612-8.
4. Maloney, W.J. and W.H. Harris, Comparison of a hybrid with an uncemented total hip replacement. A retrospective matched-pair study. J Bone Joint Surg Am, 1990. 72(9): p. 1349-52.
5. Schmalzried, T.P., M. Jasty, and W.H. Harris, Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am, 1992. 74(6): p. 849-63.
6. Schmalzried, T.P., et al., Polyethylene wear debris and tissue reactions in knee as compared to hip replacement prostheses. J Appl Biomater, 1994. 5(3): p. 185-90.
7. Muratoglu, O.K., et al., Ex vivo wear of conventional and cross-linked polyethylene acetabular liners. Clin Orthop Relat Res, 2005. 438: p. 158-64.
8. Manning, D.W., et al., In vivo comparative wear study of traditional and highly cross-linked polyethylene in total hip arthroplasty. J Arthroplasty, 2005. 20(7): p. 880-6.
9. Puri, L., et al., Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am, 2002. 84-A(4): p. 609-14.
10. Zicat, B., C.A. Engh, and E. Gokcen, Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg Am, 1995. 77(3): p. 432-9.
11. Udomkiat, P., Z. Wan, and L.D. Dorr, Comparison of preoperative radiographs and intraoperative findings of fixation of hemispheric porous-coated sockets. J Bone Joint Surg Am, 2001. 83-A(12): p. 1865-70.
12. Schmalzried, T.P., V.A. Fowble, and H.C. Amstutz, The fate of pelvic osteolysis after reoperation. No recurrence with lesional treatment. Clin Orthop Relat Res, 1998(350): p. 128-37.
13. Hozack, W.J., et al., Relationship between polyethylene wear, pelvic osteolysis, and clinical symptomatology in patients with cementless acetabular components. A framework for decision making. J Arthroplasty, 1996. 11(7): p. 769-72.
14. Hozack, W.J., P.S. Bicalho, and K. Eng, Treatment of femoral osteolysis with cementless total hip revision. J Arthroplasty, 1996. 11(6): p. 668-72.
15. Kavanagh, B.F., et al., Pelvic osteolysis associated with an uncemented acetabular component in total hip arthroplasty. Orthopedics, 1996. 19(2): p. 159-63.
16. Maloney, W.J., et al., Treatment of pelvic osteolysis associated with a stable acetabular component inserted without cement as part of a total hip replacement. J Bone Joint Surg Am, 1997. 79(11): p. 1628-34.
17. Restrepo, C., et al., Isolated polyethylene exchange versus acetabular revision for polyethylene wear. Clin Orthop Relat Res, 2009. 467(1): p. 194-8.
18. Lie, S.A., et al., Isolated acetabular liner exchange compared with complete acetabular component revision in revision of primary uncemented acetabular components: a study of 1649 revisions from the Norwegian Arthroplasty Register. J Bone Joint Surg Br, 2007. 89(5): p. 591-4.
19. Koh, K.H., et al., Complete acetabular cup revision versus isolated liner exchange for polyethylene wear and osteolysis without loosening in cementless total hip arthroplasty. Arch Orthop Trauma Surg, 2011. 131(11): p. 1591-600.
20. Massin, P., L. Schmidt, and C.A. Engh, Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty, 1989. 4(3): p. 245-51.
21. Hodgkinson, J.P., P. Shelley, and B.M. Wroblewski, The correlation between the roentgenographic appearance and operative findings at the bone-cement junction of the socket in Charnley low friction arthroplasties. Clin Orthop Relat Res, 1988(228): p. 105-9.