The cemented stem was the original THA system (Charnley stem was stainless steel).
The implant is typically highly polished Cobalt-Chrome. The high-polish seems counterintuitive (“don’t you want the cement to interdigitate into the implant?”), but remember that cement is strongest in compression, weakest in resisting shear forces. There is no interdigitation because this would emphasize shear stress, while the polished design allows the stem to compress into the cement mantle (it subsides slightly, less than a milimeter, in the first 24 hours, giving added strength). A second reason that cemented stems are smooth is that if debonding does occur, a rough surface will produce more wear particles than a smooth surface, thus accelerating osteolysis and loosening (as seen in the Exeter Stem which had a matte surface).
Collars are commonly associated with cemented stem designs as a feature to determine depth of insertion, prevent subsidence, and add stress to the calcar region to prevent stress shielding. The collar however does not prevent subsidence in the long run in a meaningful way as compared to collarless stems.
collared stem vs. collarless stem.
As discussed above, the calcar is at greatest risk from stress shielding. Could a collared stem increase stress to this region and counteract some of the disuse osteopenia. Studies demonstrate that proximal medial bone strain is 65% normal in a collarless press-fit stem, and 70-90% normal in a collared press-fit stem (however, loose fitting stem cause strains greater than a normal femur). A cemented collar also has the theoretic benefit of increasing stress transfer to the calcar region. A cemented collar also helps control height during insertion (prevents subsidence while the cement hardens). Others argue that a collar effectively prevents subsidence of the component during the initial stage after implantation which relies purely on the press-fit before ingrowth has occurred. Others argue however, that such subsidence is desirable, as a wedge fit prevents the micromotion that causes fibrous-ingrowth and persistent motion. If the collar engages the neck cut before the metaphyseal portion of the component engages, there is increased risk for fibrous-ingrowth and persistent micromotion.
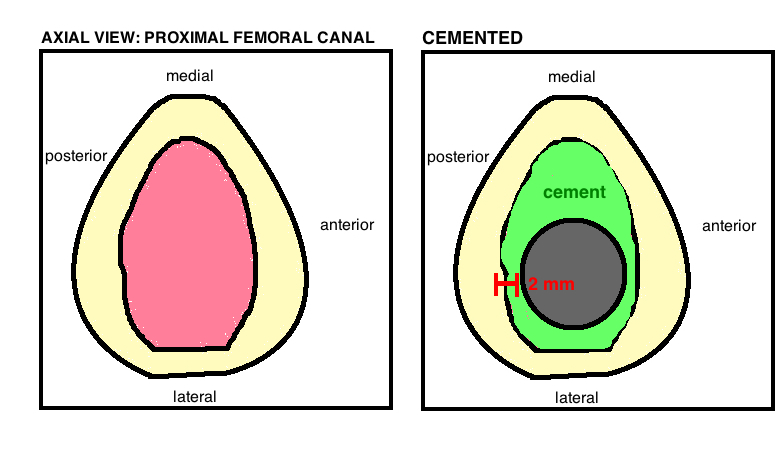
The stem itself should occupy 80% of the medullary canal. Central stem placement minimizes the chance of uneven cement mantle, preventing areas of thin cement. The target Cement Mantel is 4 mm proximally, 2 mm around the stem distally (providing sufficient thickness to prevent cement fracture). A cement centralizer affixed to the distal stem before implantation will improve the symmetry of cement mantle. The ideal length of the stem is around 13 cm (which was used by Charnley) because it allows for sufficient distal cement mantle, while a longer stem is problematic due to the native anterior femoral bow and canal narrowing near the isthmus, which together prevents a reliable cement mantle. The high modulus of elasticity in Co-Cr (in contrast to the titanium used for porous coated cementless stems) decreases proximal cement mantle stress.
Failure. When a cemented component fails, it typically fails at the component-cement interface because of debonding and then cement mantel fracture. One attempted solution was to pre-coat the stem implant with cement (the Harris Stem), however, as we now know, this design lead to a high failure rate because it emphasized shear stresses and only made matters worse at the component-cement interface.
PMMA (polymethylmethacrylate) is the bone cement used it TJA. PMMA was discovered in 1843, the first medical application of PMMA was in dentistry, and in 1945 the Judet Bros were the first to use it in orthopedics, as a femoral head prosthesis. However, its modern use (as a cement, not an implant material) was first used by Haboush in 1953, and then famously by Charnley in 1970 where it revolutionized total joint arthroplasty.
PMMA it goes by different names, such as Simplex P (Stryker brand – white color), Palacos R (Heraeus brand – green color because of chloraphyll additive) and CMWI (DePuy brand). These brand names use variations of the same base ingredients. There is an international governing body that actually regulates the standardization of biomaterials, and companies are required to comply to the mechanical and working properties of the compound (ie ultimate tensile strength, bending strength, compression strength, shear strength and fracture toughness). So whats the difference between cement brands? The final product is the same. Yet as the cement hardens, it goes thru three phases. The sticky phase, the working phase, and the hardening phase. Different cement brands have different viscosities. A high viscosity cement has a very short sticky phase and a long working phase. A low viscosity cement has a long sticky phase and short working phase.
Basic Properties.
PMMA is a polymer. Polymers have weak non-covalent bonds between side chains that break under constant strain, which leads to gradual deformation (creep) and stress reduction. This material behavior is similar to both an elastic solid and a viscous liquid thus giving the commonly used name “visco-elastic”. This property is important clinically because it indicates that implant subsidence can occur within the cement mantle, particularly during the initial few weeks after THA as the PMMA actually continues to polymerize for a few weeks in vivo (and creep is highest in fresh cement as there are more side chains that can break, leading to deformation). Furthermore, antibiotics increase the creep of cement, and actually water (which bathes the cement in vivo) increases the creep of cement. Interestingly, while the varying cement brands are uniform in their static property, there is variation in their creep due to small chemical differences. Creep is a complex process that still requires more study.
The static properties of PMMA. Poor tensile strength (25 MPa), moderate shear strength (40 MPa), and is strongest in compression (90 MPa). The modulus of elasticity for PMMA is 2400 MPa (10x less than cortical bone and 100x less than the metal implant), therefore it’s relatively soft material sandwiched between two rigid materials.
Cement Technique. PMMA is a polymer but its not the only chemical contained within the cement as its prepared. As you may know from prep on the back table, cement is made by combining a liquid and powder.
The powder contains the polymer (polymethylmethacrylate), as well as barium sulphate (or zirconium dioxide), which act as radiopacifiers, a dye (ie chlorophyll) to distinguish the cement from bone, and it also may contain antibiotics.
The liquid component contains the monomer liquid (methylmethacrylate) which allows for polymerization at room temperature. The chemical DMPT (n,n-dimethyl-p-toluidine) initiates cold curing at room temp., benzyol peroxide reacts with DMPT to catalyze polymerization, and hydroquinone (stabilizer to prevent premature polymerization).
Once the liquid and powder components are combined, they are mixed under vaccum suction to reduce the porosity and thus increase the tensile fatigue strength.
Bone preparation. Cleaning the bone (via lavage) and drying the bone (using packing or suction) maximizes cement interdigitation within the bone. Greater surface area of cement fixation improves fixation. Similarly, pressurizing the cement (using the cement gun and cement restrictor) enhances interdigitation of cement.
Stem centralization. A cement mantel is only as good as its thinnest portion (like a chain as strong as its weakest link). A stem centralizer can help obtain a uniform cement mantle to ensure the minimum 2 mm cement surrounding the implant to minimize the risk of cement fracture.
Stem Stiffness. In contrast to a press-fit stem where titanium is the preferred metal because it matches the modulus of elasticity, the cemented stem performs best when the metal is rigid and thus decreases stresses on the cement mantle. Cobalt-chrome or stainless steel are preferred materials.
Antibiotic Cement [4].
The practice of adding antibiotics to cement to either prophylax against infection or treat a periprosthetic infection began in 1970 by Buchholz and Engelbrecht [5]. An antibiotic is either added at the time of surgery or a commercial-antibiotic-impregnated-cement can be purchased off the shelf. In the USA, antibiotic cement is FDA approved for the use of revision surgery for periprosthetic infection but is not approved for use in primary TJA. The primary concern with using antibiotic cement for primary TJA is the development of bacterial resistance. Hope et al [6] demonstrated that 90% of s.aureus infections were resistant to gentimicin in the antibiotic-cement group, while only 16% were resistant if normal cement was used. However, studies demonstrate a statistically significant reduction in deep infections for primary TJA using antibiotic bone cement[7], and thus the AAOS advices for its use as prophylaxis only when considerable risk factors exist [8]. However, a randomized control trial in Sweeden, comparing systemic antibiotics (ie perioperative ancef) compared to antibiotic impregnated cement, demonstrated no significant difference for infection rate. A survey of surgeons in 2003 indicated that 12% always use antibiotic cement (primaries and revisions), 69% never used it, and 19% sometimes use it.
The properties of antibiotic release from cement varies based on the type and concentration of antibiotic. The effect of antibiotics depends on concentration, as higher concentration within the cement enables higher concentrations within the joint over a longer time [9]. Local levels of antibiotic are generally significantly higher than the minimum inhibitory concentration (significantly higher than levels obtained with intravenous antibiotics), but the ideal concentration to treat an infection is less clear…is higher concentration always associated with better clinical treatment?
Gentamicin is historically [10] the most commonly used antibiotic for cement because its family (aminoglycosides) are very thermally stable (remember that cement heats to >100° C during exothermic reaction), while also providing broad coverage and is water soluble (must be water soluble to diffuse to surrounding tissue). 2 grams of genticmin added to 40 g of the polymer has no significant impact on strength, while >4.5 g appears to significantly affect strength. This 5% concentration of antibiotics is an acceptable compromise between antibiotic strength and cement strength, although surgeons have reported increased concentrations without seeing clinically significant complications. Surgeons have reported from the Mayo Clinic, using 4 g vancomycin per 40 g batch of cement and 4.8 g gentamicin per 40 g batch of cement for their antibiotic spacers without observing toxicity in patients or cement failure.
The compressive strength to failure does not appear as significantly impacted by antibiotics as fatigue failure. Vancomycin concentrations, in contrast, have a more linear impact on the static properties of the cement. When 1, 2, or 3 g of vanco is added to cement, the cycles to failure is 90%, 70%, 50% compared to normal cement, and 1.2 or 2.4 g of tobramycin cement failed at 80% and 60% cycles compared to normal. Rifampin changes the chemical reaction of cement and slows setting-time from minutes to days, and therefore cannot be used. Furthermore, the compressive strength of cement can decrease by 40% if antibiotics are added as a liquid or if they are added to the monomer.
Multiple antibiotics in cement improves antimicrobial coverage, and may provide synergistic effects with regards to bacteriacidal effects and elution into the joint [11].
Other antibiotics have been used with varying success. Penicillins are heat-stable, however, allergy to PCN is so common that its generally avoided.
Concentration of antibiotics. On average only 5-8% of the antibiotic contained within the cement is released. Studies on tobramycin and vancomyicin suggest elution occurred over a 9 week study period, although peak levels occurred at 18 hours after implantation, and were 5x higher than levels after just 72 hours [12]. Yet, despite this seemingly rapid elution, vancomycin, due to its higher molecular weight, elutes less efficiently than aminoglycosides and therefore elutes for a longer time.
Palacos cement appears to be most efficient in releasing antibiotics, with CMW-1 releasing 24% less tobramycin and 36% less vancomycin. Palacos similarly eluted antibiotics better than Simplex cement.
REFERENCES
4. Joseph, T.N., A.L. Chen, and P.E. Di Cesare, Use of antibiotic-impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg, 2003. 11(1): p. 38-47.
5. Buchholz, H.W. and H. Engelbrecht, [Depot effects of various antibiotics mixed with Palacos resins]. Chirurg, 1970. 41(11): p. 511-5.
6. Hope, P.G., et al., Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J Bone Joint Surg Br, 1989. 71(5): p. 851-5.
7. Parvizi, J., et al., Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop, 2008. 79(3): p. 335-41.
8. Jiranek, W.A., A.D. Hanssen, and A.S. Greenwald, Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am, 2006. 88(11): p. 2487-500.
9. Masri, B.A., C.P. Duncan, and C.P. Beauchamp, Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty, 1998. 13(3): p. 331-8.
10. Wahlig, H., et al., Pharmacokinetic study of gentamicin-loaded cement in total hip replacements. Comparative effects of varying dosage. J Bone Joint Surg Br, 1984. 66(2): p. 175-9.
11. Penner, M.J., B.A. Masri, and C.P. Duncan, Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty, 1996. 11(8): p. 939-44.
12. Penner, M.J., C.P. Duncan, and B.A. Masri, The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J Arthroplasty, 1999. 14(2): p. 209-14.